Transitional Arrangements and FAQs
UK Biobank introduced new Access Fees and a new Material Transfer Agreement (MTA) in 2021.
New Access Fee structure
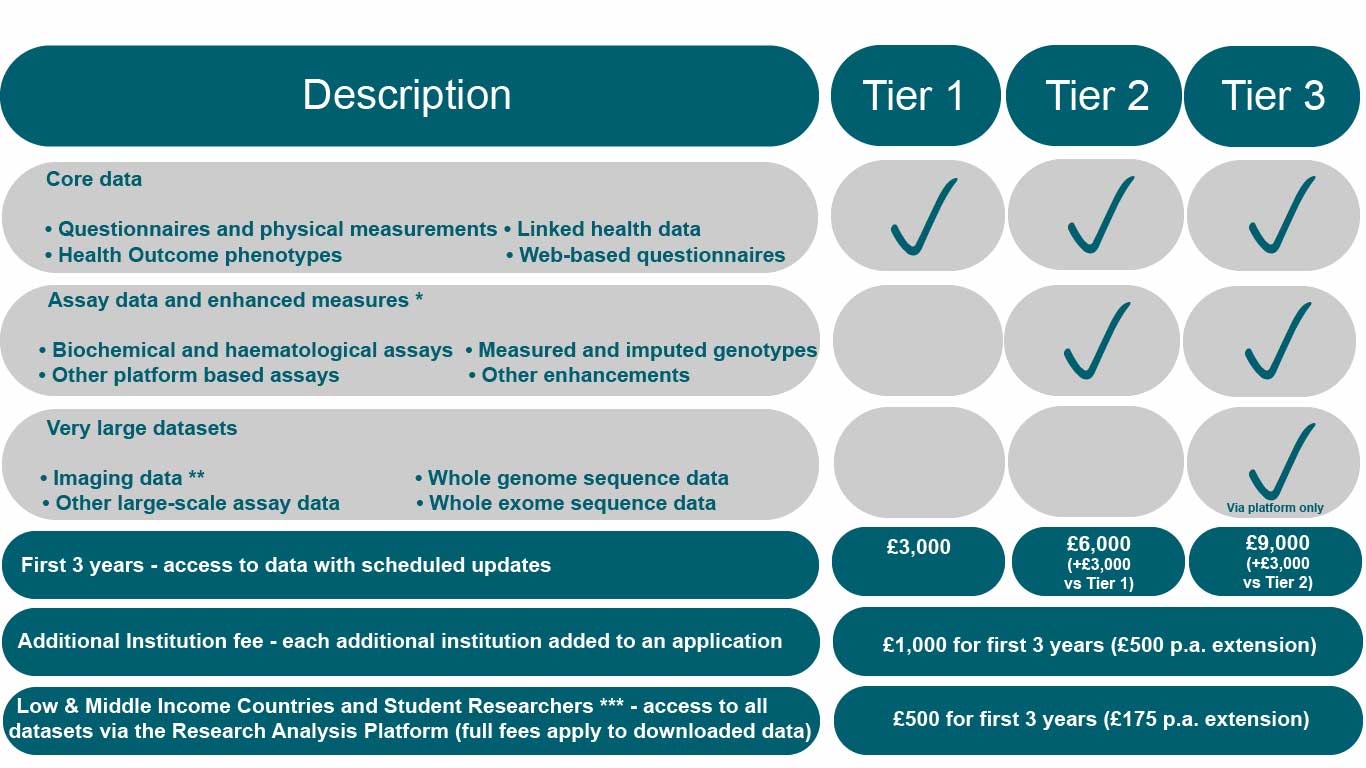
* These data are available via the Research Analysis Platform at no additional cost (over and above the Tier 1 payment).
** The imaging data are available for download with the Tier 3 payment: but at no additional cost over and above the Tier 2 payment via the Research Analysis Platform in 2022.
*** Applications from Student Researchers must be for the sole purpose of performing a postgraduate student project (e.g. MSc or PhD or equivalent), submitted by the student or their supervisor.
Researchers should use the information below to identify which scenario their research project fell into on March 31st 2021 and read the corresponding FAQs for more information about how the transitional arrangements affect their research project.
Scenario 1: This scenario applied to a research project which had reached its project completion date and the MTA for the research project had expired. The MTA expired 12 months after the project completion date.
Scenario 2: This scenario applied to a research project with a project completion date on or before 31st March 2021 and a current MTA. The MTA expired 12 months after the project completion date.
Scenario 3: This scenario applied to a research project which had not yet reached its project completion date and had a current MTA. This included research projects with a project completion date after 1st April 2021.
Scenario 4: This scenario applies to a research project which was submitted (but has not yet been approved or started) on or before 31st March 2021.
Scenario 5: This scenario applied to a research project which was submitted on or after 1st April 2021.
How to find the completion date or MTA expiry date for a research project:
The current project completion date can be viewed by clicking the “live link” referenced within Annex A or Annex 4 of the MTA, located in the Project ‘Documents’ in AMS. Under old versions of MTA, the expiration date of the MTA is 12 months after the agreed project completion date.
UK Biobank automatically updated the project completion dates for applicable research projects in accordance with Scenario 3 below before AMS re-opened in June 2021.
Transitional Arrangements FAQs
Scenario 1 (expired projects)
The Principal Investigator (PI) is required to ensure that by the 31st August 2021 (at the latest):
- the findings from the research project are in the public domain (e.g. published in an established journal, an online journal or accessible on a suitable website);
- associated results (in a suitable and comprehensible format, with such explanatory documentation as may be needed) are returned to UK Biobank;
- UK Biobank data are deleted (or made permanently inaccessible); and
- written confirmation that all of the above has been done is sent to UK Biobank by email to access@ukbiobank.ac.uk.
No changes of any type are permitted to the research project. Any further research activity will require a new MTA and payment of the applicable new Access Fees for the minimum 3 year period.
If a research project has expired, but the PI wishes to continue with the same research project as before (i.e. the same project, same scope), the PI will need to notify UK Biobank via AMS messaging or via email to access@ukbiobank.ac.uk. The PI and Lead Collaborators (if applicable) will then be issued with the new MTA and payment of the applicable new Access Fees for the minimum 3 year period will be required
Scenario 2 (expired projects with current MTA)
The Principal Investigator (PI) is required to ensure that by the MTA expiry date or 31st August 2021 (whichever is latest):
- the findings from the research project are in the public domain (e.g. published in an established journal, an online journal or accessible on a suitable website);
- associated results (in a suitable and comprehensible format, with such explanatory documentation as may be needed) are returned to UK Biobank;
- UK Biobank data are deleted (or made permanently inaccessible); and
- written confirmation that all of the above has been done is sent to UK Biobank by email to access@ukbiobank.ac.uk.
No changes of any type are permitted to the research project. Any further research activity will require a new MTA and payment of the applicable new Access Fees for the minimum 3 year period.
If a research project has reached its completion date but the PI wishes to continue with the same research project as before (i.e. the same project, same scope), the PI will need to notify UK Biobank via AMS messaging or via email to access@ukbiobank.ac.uk. The PI and Lead Collaborators (if applicable) will then be issued with the new MTA and payment of the applicable new Access Fees for the minimum 3 year period will be required
Scenario 3 (current projects)
If no substantive changes are required to a current or already submitted application, then the research project will continue under the original MTA and the original Access Fee structure. The findings from the research project must be published and any results (in a suitable and comprehensible format, with such explanatory documentation as may be needed) returned by the MTA expiry date.
Automatic extension: in order to allow existing projects to complete before the new MTA and new Access Fees become applicable, the following extensions to a project’s duration have been automatically applied by UK Biobank to projects that have a completion date on or after 01/04/2021 but before 31/12/2021, at no additional cost to the research project:
- Research projects with a project duration of less than 3 years have had the duration increased to 3 years; and
- After taking into account the automatic extension in 1 above, research projects with a completion date between 01/04/2021 and 31/12/2021 have had the completion date set to January 2022.
If an existing research project has a duration of longer than 3 years that has already been agreed with UK Biobank, or a completion date after 31/12/2021, then this will remain unchanged.
UK Biobank will not send individual notifications to researchers about these revised completion dates. However, researchers can find their revised completion date in AMS by clicking the "live link" referenced within Annex A of the original MTA, located in the Project 'Documents' in AMS. The automatic extensions explained above have now been applied.
If UK Biobank determines that the proposed change is consistent with the original research project and the project scope has not already been extended on multiple occasions, then this will be permitted. No additional Access Fees will be charged and the research project will continue under the original MTA.
Changing the PI (or Lead Collaborator at a Collaborating Institution) to another approved researcher at the PI’s Institution (or Collaborating Institution) will be permitted. No additional Access Fees will be charged and the research project will continue under the original MTA.
However, if the PI will be at an Institution which does not have an MTA as part of the relevant research project then that new Institution will require a new MTA. Moreover, so too will all Collaborating Institutions working on that research project because researchers working on the same project cannot be on different versions of the MTA.
Each Institution will have 90 days to sign up to the new MTA. Payment of the new ‘Additional Institution Fee’ (£1,000) will be charged to the Applicant Institution, which will be responsible for the payment of the fee (although UK Biobank will accept payment from a Collaborating Institution).
Requests for a duration extension that is outside the scope of the automatic extensions set out above, will only be considered during the final year of the project (i.e. within one year of the completion date). Duration extensions of up to 3 years can be requested in 1-year increments.
Duration extensions will be subject to approval by UK Biobank and will require a new MTA and payment of the applicable new Access Fees (pro-rated where applicable, based on the Access Fees at the time).
The new Collaborating Institution will be considered in the normal way as to whether the researcher is bona fide. However, if approved, the Applicant Institution and all Collaborating Institutions working on that research project, will be required to enter into a new MTA because researchers working on the same project cannot be on different versions of the MTA.
Each Institution will have 90 days to sign up to the new MTA. Payment of the new ‘Additional Institution Fee’ (£1,000) for each new Collaborating Institution will be charged to the Applicant Institution, which will be responsible for the payment of the fee (although UK Biobank will accept payment from a Collaborating Institution).
UK Biobank periodically updates certain core data (such as health linkage data).
These updated data will be available to the project at no additional cost, and the project may continue under the original MTA.
If the original bulk* Access Fee of £500 has already been paid by the researcher, then updated bulk data will be available with no requirement for a new MTA and no additional Access Fees will be charged.
If the original bulk Access Fee has not been paid by the researcher, then a request by the researcher to add these data will require a new MTA and payment of the applicable new Access Fees.
* For the definition of “bulk” data, please see: https://biobank.ctsu.ox.ac.uk/~bbdatan/Data_Access_Guide.pdf
Core data: If the additional data would have formed part of the core data (from the original application) then these data will be available without the requirement for a new MTA and the payment of new Access Fees.
Bulk data: If the additional data would have comprised the original ‘bulk’ data (broadly data in the new Tier 3 categorisation) then, as long as the bulk data fee has already been paid, the researcher may have access to any of the new data (subject to the exceptions below). If the bulk data has not been paid, then this request is a change which requires a new MTA and payment of the new Access Fees.
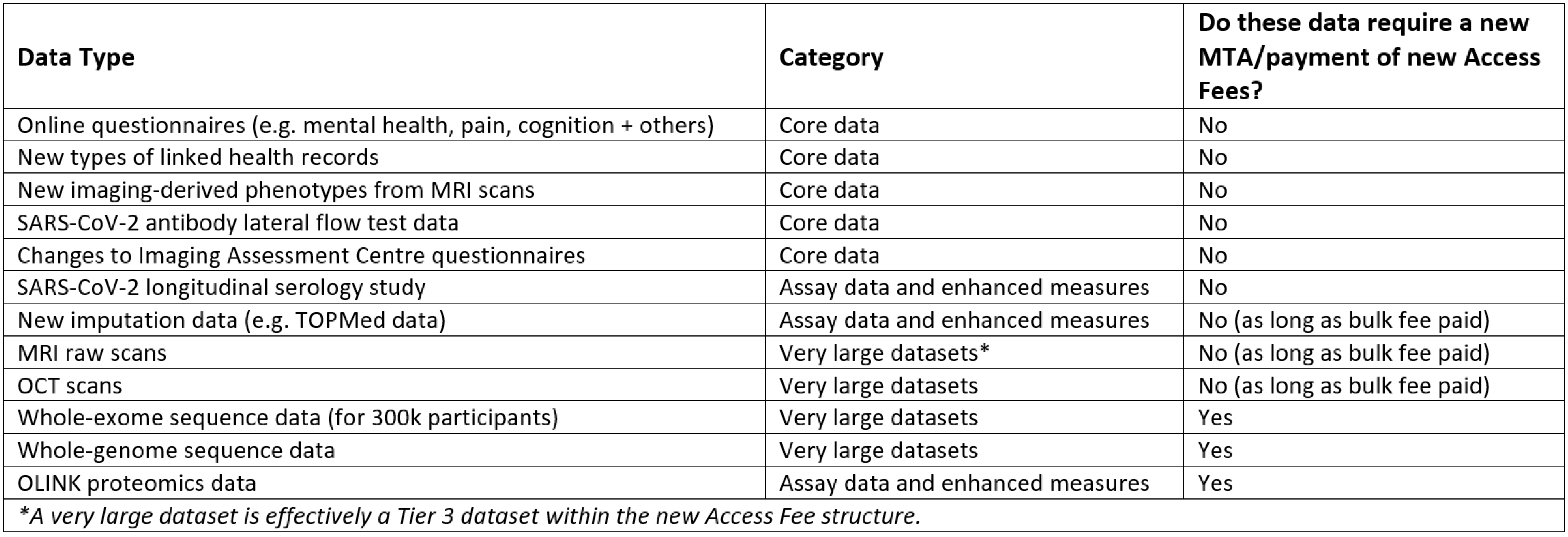
New data: With certain exceptions (see the table below), new data released by UK Biobank is considered part of an existing research project with no requirement for a new MTA and no additional Access Fees. The data that will, for all researchers, require a new MTA and payment of the applicable new Access Fees, are currently as follows:
- Subsequent releases of additional 300k Exome Sequencing data
- Whole Genome Sequence data
- OLINK proteomics data
UK Biobank will exercise reasonable discretion (based on size and type of data, and in accordance with the approach set out above) as to whether new data (in addition to those listed above) will require a new MTA and payment of the new Access Fees. Please see table 1 below for more information.
Table 1
Scenario 4 (new application submitted but not approved or started on or before 31st March 2021)
The application will be assessed by UK Biobank using the original Access Procedures. If the application is approved, the original Access Fees will be charged and the original MTA will be issued. The project duration will be set to 3 years.
Any subsequent changes to the research project will be dealt with in the same way as Scenario 3 above.
Scenario 5 (new application submitted on or after 1st April 2021)
The new application will be assessed by UK Biobank using the new Access Procedures (please note that the underlying access criteria are unchanged) and, if approved, will be subject to the new MTA and payment of the applicable new Access Fees. Please see the FAQs below for more information.
General FAQs (what are we doing, why and why now)
The last project completion date can be viewed by click the "live link" referenced within Annex A or Annex 4 of the MTA, located in the 'Documents' tab.
Under the old versions of the MTA, the expiration date of the MTA is 12 months after the agreed project completion date.
UK Biobank has automatically updated the project completion dates for applicable research projects as explained in the Scenario 3 section of these FAQs above.
No, but the researcher is able to view their project’s revised completion date in the AMS system.
Please see the FAQ above for information on how to determine the completion date or MTA expiry date of a research project.
No. UK Biobank’s approach remains as it was when it first opened for access, namely that the resource is available to all bona fide researchers for health-related research that is in the public interest. No distinction is made between researchers, whether they are academic or commercial, domestic or international.
As before, access to the UK Biobank resource is subject to application and approval by UK Biobank, and the criteria for assessing access applications are unchanged. The Access Procedures have been updated to reflect changes in current practice and are available here.
UK Biobank has been open for access applications since 2012. During that time, the scale and scope of the data (including baseline, assay, health-record, imaging and genetic data) have grown substantially. It is, therefore, considered appropriate at this time to adjust the Access Fees to reflect these changes.
UK Biobank’s new Access Fees remain based on the underlying principle of seeking to recover only the costs of processing/managing an access application (and not any of the costs of creating or maintaining the resource). The new Access Fees reflect the increasing costs (including inflation) to UK Biobank of making the increasingly large amounts of increasingly complex data available to researchers.
The structure of the Access Fees has also been modified, as shown in the new Access Fee structure above. The original two-tier fees (fees for core, £1,750, and bulk data, £500) have been replaced by three tiers of fees, broadly based on the size of the respective datasets.
Further, there is a transition to a periodic rather than a one-off charge: namely to an initial 3-year period, which is renewable for further periods of 1, 2 or 3 years. This is being done to give researchers a more consistent time-frame in which to conduct their research. It also reflects the costs of providing updates of data and extensions to scope and duration of projects sought by significant numbers of researchers, which were previously not recovered.
The MTA is changing for two reasons: firstly, to bring it in line with current access practice and, secondly, to address new regulatory and legal requirements that have been introduced in recent years.
As before, UK Biobank’s MTA is not negotiable. UK Biobank considers it to be a fair and balanced agreement and has revised certain provisions in response to researcher feedback over the years. Moreover, UK Biobank does not have the resources to negotiate the MTA with each researcher and (even if it did) UK Biobank considers – on the basis of transparency, equity and fairness – that the same contractual arrangements should be provided to all researchers.
The new MTA will place renewed emphasis on the submission of Annual Project Reports by the Applicant PI for a research project and introduces annual confirmation of compliance with the provisions of the MTA (including but not limited to the data protection and security requirements). Lead Collaborators will not be required to provide project updates but must submit an Annual Confirmation Form to confirm their compliance with the provisions of the MTA on an annual basis.
Non-compliance or failure to submit the Annual Project Report and the Annual Confirmation Form on time would lead to researchers not being able to obtain access to additional data, and persistent non-compliance may lead to research projects being terminated and future applications being declined.
The Access Procedures have been updated to reflect changes in current practice and are available here. Similarly to the MTA, the Access Procedures have been updated to reflect the changes we’ve made over time to the research application process. However, it should be emphasised that the underlying approach to access remains the same: namely that UK Biobank treats all applications – whether from academic, commercial or other organisations, whether domestic or international – in the same way and subject to the same standards and same access criteria.
UK Biobank has two sets of reduced fees https://www.ukbiobank.ac.uk/enable-your-research/costs : one for postgraduate students and one for principal applicants from Low and Middle Income Countries (LMIC), a list of which is available here.
The new Research Analysis Platform (see below) will considerably facilitate access by allowing researchers to go to the data, rather than the researcher needing to have local storage and compute capacity to download and use the data in a secure environment.
Access Fees FAQs
Please see the new Access Fee structure above for more information on the new Access Fees.
Researchers can elect to extend their research project – subject to being compliant with UK Biobank’s reporting and annual confirmation requirements – in the final year of their initial 3 year term. This extension can be requested for incremental periods of 1-year, so that an extension of 1 or 2 or 3 years may be requested and payment is pro-rated accordingly (based on the Access Fees at the time). Further, if applicable (and subject to equivalent criteria) researchers can extend their research project for a further 3-year period (i.e. to take the duration from 6 to up to 9 years and so on) on an equivalent basis
If access to an additional Tier is requested once a project is underway then the fee charged will be pro-rated based (in rounded-up years) on the remaining project duration. For example, if a project with 2 years left on the term that is seeking expanded access to Tier 2 data (where the additional Access Fee is £3,000) then the additional charge will be £2,000 (being 2/3rds of £3,000).
The ‘Additional Institution Fee’ per Collaborating Institution is to cover UK Biobank’s administrative costs of adding an institution to an application. A fee of £1,000 is charged for each additional Collaborating Institution. This fee is not pro-rated based on the remaining project duration because the work required is the same irrespective of the duration. All payment requests default to the Applicant Institution, but the UK Biobank will accept payment from a Collaborating Institution.
There are certain data – set out in the new Access Fee structure above – where access can be obtained through the RAP at no extra cost, which is intended to encourage its use.
Applications from bona fide postgraduate students will be considered for the reduced Access Fee where the following criteria are met:
- The application is submitted by a student or their supervisor for the sole purpose of performing a postgraduate student project (e.g. MSc, PhD or equivalent). Authorship of the resulting paper must be led by the student;
- The application cannot be used to conduct research for any other purpose, nor can it be used for multiple student (or other) projects; and
- Any collaborators must have a clearly articulated and relevant role in of the student’s research project (and will be permitted only at UK Biobank’s discretion).
A reduced Access Fee of £500 for the first 3 years (£175. p.a. extension) is payable – which is not pro-ratable - and provides access to all data via the RAP only (full Access Fees apply to downloaded data) and where there are researcher credits available (see FAQs on the RAP below).
Applications from low and middle income countries will be considered for the reduced Access Fee.
A reduced Access Fee of £500 for the first 3 years (£175. p.a. extension) is payable and provides access to all datasets via the RAP only (full Access Fees apply to downloaded data).
Material Transfer Agreement (MTA) FAQs
No. The underlying obligations of the researcher to UK Biobank under the new MTA are equivalent to those under the original MTA: namely that a researcher is obliged to publish their findings and to return their results. As before, the provisions covering Intellectual Property are effectively the same: researcher inventions remain the property of the researcher.
The underlying restrictions on the researcher are also equivalent to those under the original MTA: namely that UK Biobank data cannot be sub-licensed, published or otherwise made available to third parties, and that a researcher cannot prevent or inhibit other research (e.g. by patenting), which are equivalent to those in the original MTA
More emphasis will now be placed on Applicant PI’s submitting Annual Project Reports. The purpose of the Annual Project Report is for the Applicant PI to provide UK Biobank with:
- An annual progress update on the research project, including details of research output, publications and plans for the next 12 months; and
- Annual confirmation that the research project still complies with the provisions of the MTA (including but not limited to the security and data protection requirements).
Lead Collaborators will also be required to submit an Annual Confirmation Form to confirm on an annual basis that they are still complying with the provisions set out in the MTA (including but not limited to the security and data protection requirements). Lead Collaborators will not be required to provide annual project progress updates (which is the responsibility of the Applicant PI).
Non-compliance or failure to submit the Annual Project Report and the Annual Confirmation Form on time would lead to the project not being able to access additional data and persistent non-compliance may lead to research projects being terminated and future applications being declined. The presumption should be – only rebutted if there is very good reason to the contrary – that non-compliance will lead to the above access restrictions.
Please see the specific FAQs below in relation to data protection for further information.
When a new MTA is issued by UK Biobank, it will be sent to the relevant researchers for signature via DocuSign. The relevant researchers will receive an email which contains a link to view and sign the new MTA in DocuSign. Researchers do not need to create a DocuSign account in order to view and sign the new MTA.
Yes. Researchers working on the same project must be on the same version of the MTA.
Therefore, if an Applicant Institution makes a change to a research project which results in them requiring the new MTA, all Collaborating Institutions working on the same research project must also agree to the new MTA.
If a new Collaborating Institution is added to the research project under the new MTA, all other Institutions (including the Applicant) working on the research project must also sign the new MTA.
Each Institution will have 90 days to sign the new MTA
UK Biobank has updated clause 3.4 of the Applicant Data Only MTA (clause 3.5 of the Data & Samples MTA) to clarify the obligation on researchers to confirm that their Findings and Results Data do not infringe the rights of any third party. As UK Biobank makes researcher Findings and Results Data available to other researchers, it considers that it is reasonable for a researcher to take a certain amount of care and diligence in the preparation of these materials (so that, for example, without suitable permission, a researcher cannot simply copy the results or workings of another researcher and return them to UK Biobank). This obligation on researchers is qualified in the following manner:
- The confirmation is given at the time, respectively, of the publication of the Findings or the return of Results Data (in other words it is a one-off confirmation);
- The confirmation is given to the best of knowledge and belief of the researcher (in other words it is not unqualified);
- The confirmation does not require a researcher to conduct external patent searches (as UK Biobank does not consider it necessary or commensurate, given that UK Biobank’s principle concern is to ensure that Findings and Results Data are the original work of the researcher).
The approach set out in the updated clause (as elaborated upon in this note) is the approach that UK Biobank will adopt to MTAs executed both before and after this date.
Research Analysis Platform (RAP) and Downloading FAQs
The RAP is a cloud-based platform providing a research environment that will allow researchers to access UK Biobank’s data without the need to download the data. It provides access to storage and compute resources that allow researchers to undertake their analyses within the platform.
A RAP user guide is available here.
The RAP is now available. Click here to sign up.
The RAP will contain several tools to allow analysis across the various modalities within the UK Biobank datasets. Click here to view the RAP toolkit for more details.
For researchers undertaking an approved project, there is no additional charge by UK Biobank to access the RAP. However, every researcher who uses the RAP will have to accept certain standard terms and conditions of usage with the platform provider (DNAnexus). The main dataset is stored in the AWS cloud on the London node (EU-West-2).
There is no upfront charge to researchers using the RAP, although certain activities may incur a fee (payable to the platform provider), for example there are fees for storage of uploaded or derived data, compute and analysis (dependent on instance type) and egress charges.
The full rate card, which may change from time to time, can be found here.
On opening an account on the RAP, researchers will be provided with £40.00 worth of credits by the platform provider to explore the RAP.
A grant application process will be available in Q3 2021 to provide postgraduate student researchers, early career researchers and researchers from LMIC with researcher credits to be used in the RAP. This is provided in conjunction with Amazon Web Services (AWS).
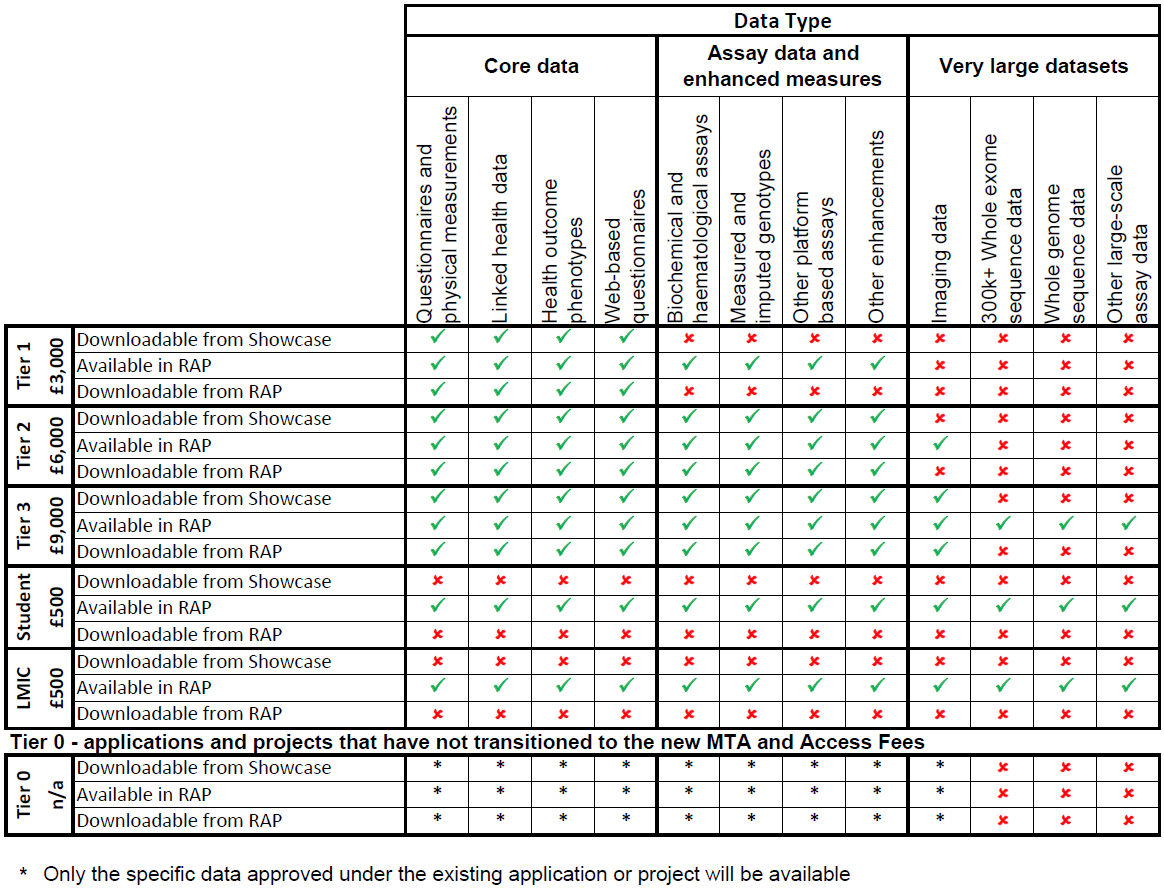
All data is available within the RAP, but access to data (and the ability to download data) depends on the Tier Access Fee paid. There are also certain restrictions on downloading from the RAP. Please see table 2 below for further information.
The ability of researchers to download data will be (generally) dependent on the Tier Access Fee paid.
It should be noted all sequencing data (both Whole Genome and Whole Exome) is only available in the RAP and is not available to download.
Yes, as long as the data imported into the RAP is to enable research in line with your research project.
There is no limit on the size of data (save that the cost of data storage to the researcher will increase according to the amount of data stored in the RAP) or the type of data that can be imported into the RAP. Data must be used in line with the UK Biobank’s MTA and the RAP terms and conditions.
There are several tools directly available within the RAP, principally based on the use of RStudio and Jupyter notebooks with support for varying scripting languages (including STATA, R and Python). The Python-enabled notebooks include matplotlib, numpy, pandas and SciPy libraries, and with the ML-enabled notebook including support for TensorFlow and nipype imaging processing libraries.
At this time, support for statistical tools is via the use of RStudio and Jupyter notebooks with support for STATA, R and Python. The ability to use other statistical software, such as PSPP, is under consideration for inclusion in future platform releases as part of the development roadmap. The researcher (or software owner) is responsible for obtaining any applicable licences and for ensuring that the use of the software on the RAP conforms to the terms of the licence agreement.
The RAP provides support for researchers to import their own toolchains (provide they adhere to certain technical standards) and also provides for the use of Jupyter Lab notebooks with support for custom code, such as scripted in Python and R. Support for other languages are on the development roadmap.
However, the RAP itself cannot be modified with custom code. The RAP is a standard product and any enhancements may be considered as future roadmap items.
No, all code, analysis and results are not accessible to anyone outside your workspace; that said, if a researcher wishes to make its code, example notebooks or tools public this is possible. When a researcher has completed its analysis, there is an option to return data via the RAP directly to UK Biobank.
Table 2
Data Protection FAQs
This note sets out some Frequently Asked Questions in relation to UK Biobank's approach to the data protection framework that it has adopted. These FAQs are for general information purposes only. UK Biobank is not providing any legal advice to researchers who should seek their own legal advice, particularly in relation to compliance with any domestic law matters.
Terms used in this data protection section of the FAQs have the same meaning as in the MTA.
UK Biobank continues to go to extensive efforts to de-identify the data it releases to Applicant and Collaborator researchers (referred to in these FAQs as the researcher). Nevertheless, it is possible that certain of the UK Biobank datasets (in particular, more detailed data such as Whole Genome Sequences) may be considered by some regulatory authorities to contain personal data.
In this regard, UK Biobank requires the researcher to treat UK Biobank data as if the researcher is a controller of the UK Biobank data and as if the UK Biobank data is personal data. The specific requirements on the researcher contained in the new MTA are a direct function of these requirements.
The relevant provisions in the new MTA are necessary in order for the parties to adhere to the prevailing legislation in the UK (which is the UK GDPR and the Data Protection Act 2018). Compliance with these provisions will enable UK Biobank to provide the data to the researcher.
UK Biobank considered that the adoption by the UK of the GDPR into domestic law, and the implications of the UK leaving the EU, makes it an appropriate time to update the provisions relating to data in the MTA.
The UK Data Protection Act 2018 (DPA) sets out the framework for data protection law in the UK. It came into effect on 25 May 2018. It sits alongside the UK GDPR, and impacts on how the UK GDPR applies in the UK.
The GDPR is the General Data Protection Regulation (EU) 2016/679. It sets out the key principles, rights and obligations for the processing of personal data. It came into effect on 25 May 2018. As a European Regulation, it had direct effect in UK law and automatically applied in the UK until the end of the Brexit transition period. The GDPR now forms part of UK law under the European Union (Withdrawal) Act 2018, with some technical changes to make it work effectively in a UK context and is now referred to as the “UK GDPR”.
In addition, specific EU Exit Regulations[1] (aka Brexit) amend the GDPR, the DPA and other privacy regulations so that these laws can continue to work properly in a context where the UK is no longer an EU Member State.
[1] The Data Protection, Privacy and Electronic Communications (Amendments etc.) (EU Exit) Regulations 2019 (SI 2019/419), as amended by the Data Protection, Privacy and Electronic Communications (Amendments etc.) (EU Exit) Regulations 2020 (SI 2020/1586)
Personal data, as defined in the UK GDPR, means any information relating to an identified or identifiable natural person. An identifiable natural person is one who can be identified, directly or indirectly, in particular by reference to an identifier such as an identification number or to one or more factors specific to the physical, physiological, genetic, mental, economic, cultural or social identity of that natural person.
UK Biobank goes to significant lengths to de-identify the data it releases to researchers, by removing direct and indirect identifiers, such that (even taking into account publicly available information) it should not be possible for a researcher to be able to re-identify a Participant. Further, under the terms of the MTA, researchers are expressly prohibited from actual (and making any attempts towards) re-identification.
The UK Biobank data released to researchers is de-identified and comes with a so-called pseudo-identifier (known as the Encoded ID or EID): the EID is a number algorithmically generated by UK Biobank and uniquely assigned to each research project. This can be reversed by UK Biobank but not by the researcher. The reason for this is that otherwise results and derived variables generated by researchers (which are of considerable utility to UK Biobank and to other researchers) could not be linked back at a participant level. This is why UK Biobank does not fully anonymise the data as that would prevent such linkage.
The data which UK Biobank releases are de-identified such that it should not be possible for a researcher to be able to re-identify a Participant. However, it is possible that certain of the UK Biobank datasets (in particular, more detailed data such as Whole Genome Sequences) may be considered by some authorities to contain personal data.
UK Biobank refers to this data in the new MTA as ‘Participant Level Data’.
A controller determines the purposes and means of the processing of personal data.
A processor processes personal data on behalf of the controller. The processor must act on the instructions of the controller and does not have the discretion to determine the purposes and means of processing of the personal data.
UK Biobank has specific rules which enable a researcher to use Third Party Processors subject to the provisions set out in the new MTA.
UK Biobank considers that a researcher is a controller of the Participant Level Data.
Although the scope of the research project is approved by UK Biobank, the researcher (rather than UK Biobank) determines the specific purposes and means of the processing by which the Participant Level Data are used in order to conduct its Approved Research Project.
Even though the researcher is a separate and independent data controller of the Participant Level Data, its use and processing of the Participant Level Data must be strictly in accordance with the Approved Research Project, Permitted Purpose and the provisions of the new MTA.
No. Under the UK GDPR, two or more controllers are "joint controllers" when they jointly determine the purposes and means of processing. As the Approved Research Project is conducted by the Researcher independently of UK Biobank, there is no “joint” activity and, consequently, UK Biobank and the researcher are not joint controllers.
No. Processors have to accept strict controls to act in accordance with the instructions of the controller. As the researcher needs to conduct the research (and not UK Biobank), they must determine the means and purposes of the processing in relation to the Participant Level Data. They are not under the instruction of UK Biobank, and thus are not acting as a processor in the context of a research project.
The UK GDPR restricts the ability of a data controller (such as UK Biobank) to transfer personal data outside of the UK, unless the rights of individuals in respect of their personal data are protected in another compliant way. The issue of personal data is covered in a separate FAQ above. In accordance with the UK GDPR, UK Biobank will permit transfers to researchers located outside the UK in two circumstances: (i) to researchers located in a country covered by an "adequacy regulation" or (ii) in accordance with approved standard contractual clauses, such as EU Standard Contractual Clauses or the International Data Transfer Addendum to the EU Commission issued by the UK Information Commissioner (“UK Addendum”).
Adequacy Regulations: Adequacy determinations are made by the UK government and currently the territories covered are the EEA, EU or EEA institutions, bodies, offices or agencies, Andorra, Argentina, Canada (commercial organisations), Faroe Islands, Gibraltar, Guernsey, Isle of Man, Israel, Jersey, New Zealand, Japan (private sector organisations), Switzerland or Uruguay. These territories may be updated by the UK Government from time to time both to add or remove territories from the relevant list. If the researcher is located in a territory covered by an adequacy regulation then the transfer of personal data from the UK to these countries is permitted and no further action is required.
EU Standard Contractual Clauses/UK Addendum: If the researcher is not located in one of the listed countries then approved standard clauses will automatically apply to any such transfer. Historically, the standard clauses were the Controller-to-Controller EU Standard Contractual Clauses and this is what UK Biobank has applied to date. Due to the UK leaving the EU, the UK Information Commissioner’s Office (“ICO”) has produced a separate set of standard clauses, called the UK Addendum, and these will deemed to be incorporated (as necessary) into the new version of the MTA issued from the end of July 2022 onwards. The new clause provides for the mechanism for this incorporation and no further action – other than familiarisation with the UK Addendum by the researcher – is required.
In UK Biobank’s view, the EU Standard Contractual Clauses and the UK Addendum create broadly comparable obligations on the researcher, although the researcher is advised to review the relevant obligations and seek their own advice and counsel.
Researchers currently using the EU Standard Contractual Clauses – under the ICO’s transitional provisions (https://ico.org.uk/media/for-organisations/documents/4019534/scc-transitional-provisions.pdf) - are permitted to continue to use these clauses until 21 March 2024. For research projects which may extend beyond 21 March 2024 and are currently using the EU Standard Contractual Clauses, UK Biobank will issue a further announcement at the end of 2023 which will provide for an automatic change from the EU Standard Contractual Clauses to the UK Addendum.
It should be noted that neither the EU Standard Contractual Clauses nor the UK Addendum are negotiable and automatically invalidated by any change.
As a controller, the researcher must comply with their obligations under the Data Protection Legislation in relation to the Participant Level Data.
To the extent that the UK GDPR applies to the researcher as a controller, more information can be found on the UK Information Commissioner's Office website here: https://ico.org.uk/ and here: https://ico.org.uk/for-organisations/guide-to-data-protection/guide-to-the-general-data-protection-regulation-gdpr/controllers-and-processors/what-does-it-mean-if-you-are-a-controller/#1
For researchers with whom Controller-to-Controller Standard Contractual Clauses are required, the researchers must also comply with importer obligations and the data protection principles set out in Annex A of the Standard Contractual Clauses.
No. Any and all contact with individual participants must only ever be undertaken by UK Biobank.
There is no realistic prospect that a researcher would be contacted directly by a UK Biobank Participant. However, should this happen (or appear to happen, as a researcher has no means of establishing whether an individual is or is not a Participant and is in any event prohibited to trying to re-identify or contact Participants) the researcher should not engage with the Participant and instead refer the enquiry to UK Biobank.
UK Biobank will introduce restrictions which will enable researchers to use the Whole Genome Sequence data and (from now on) the Whole Exome Sequence data on the Research Analysis Platform only. It will still be possible for the other UK Biobank data to be downloaded by a researcher, as before in a de-identified form.
Last updated
