Information sheet: Phase 1
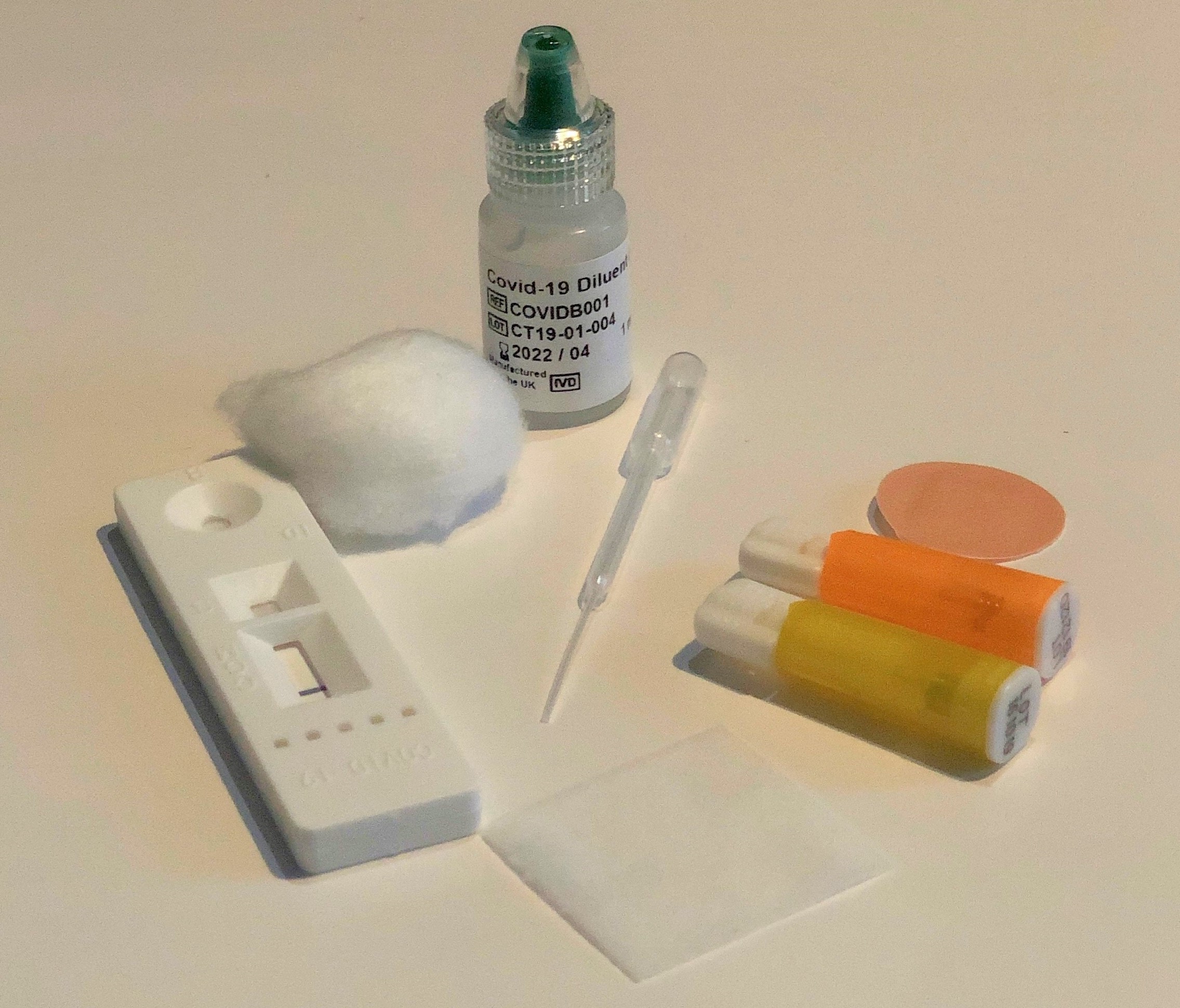
The phase 1 study uses the Fortress Fast test kit
Information sheet: Phase 1 (Fortress Fast COVID-19 Home Test)
We have invited you to help UK Biobank undertake urgent research on the SARS-CoV-2 coronavirus that causes COVID-19 by taking part in this new study; its findings will provide researchers with unique opportunities to investigate the long-term health effects of SARS-CoV-2 infection (“Long COVID”) and other health research related to COVID-19. The study is taking part in two phases, and you have been invited to participate in phase 1 which is running from February to March 2021. All remaining UKB participants will then be invited to take part in Phase 2 of the study, which will be starting in mid-March 2021.
The study involves doing a simple antibody test (the Fortress Fast COVID-19 Home Test) at home, which will tell you if it is likely that you have previously been infected with the coronavirus. If you agree to help, we will send you a finger-prick home-testing kit to find out if you have antibodies to the coronavirus. Taking the test is simple and quick to do and you can let us know the result via the UK Biobank participant website.
Taking part is entirely voluntary and will not affect your ongoing relationship with UK Biobank. Please take the time to read this information sheet carefully. It explains why we are asking you to help and what it would involve. If anything is not clear, or if you would like more information, please refer to our frequently asked questions, telephone our Participant Resource Centre free of charge on 0800 0 276 276 (Monday to Friday 9am to 5pm), or email us at ukbiobank@ukbiobank.ac.uk
Thank you for your continued support
This research study aims to measure the extent of SARS-CoV-2 (coronavirus) infection in the UK Biobank population by measuring blood antibodies to the virus. This information will enable researchers to determine the genetic and clinical risk factors for COVID-19, explore factors which influence COVID-19 disease severity and understand the long-term health effects of SARS-CoV-2 infection (“Long COVID”). This research may, in turn, lead to better ways to manage the disease. We are inviting all UK Biobank participants to use a home self-testing kit that measures the presence of antibodies to the coronavirus from a finger-prick drop of blood. This test provides an indication as to whether you have been previously infected with the coronavirus. We will also ask you to complete a short online questionnaire to let us know your test result.
You have been invited because you are a participant in UK Biobank. Please note that this study is different from UK Biobank’s coronavirus study involving the collection of repeated blood samples for 6 months. Even if you took part in that study, we would still like you to take part in this new study.
No; whilst we are keen for all of our participants to contribute, your participation is entirely voluntary. We do understand that you may not be able or wish to help and this will not affect your future relationship with UK Biobank.
We will ask you to:
- Use a home antibody self-testing kit (Fortress Fast COVID-19 Device), which we will deliver to your home. This kit uses a small drop of blood from a finger-prick to measure the presence of blood antibodies to the SARS-CoV-2 virus. It will provide you with a result in 10 minutes.
- Log into the UK Biobank participant website to let us know the result of the test and whether you have had a COVID-19 vaccination.
- If your test indicated that you have the antibodies (a “positive” result), and you also tell us that you have not been vaccinated, we will ask you to repeat the test to double-check the result. We will send you a second kit within a couple of weeks of receiving your first test result.
If you test positive and you have not yet received a COVID-19 vaccination, we would like you to take a second test (that we will send to you when you tell us your first result). This is to make sure that your test result is truly positive. This is important as we want to reduce the number of false positives as much as possible (i.e. those who report as positive but are actually negative).
If you test positive and you have received a COVID-19 vaccination, you may be invited to take part in a further study which will allow us to see if the antibodies are a result of your vaccination or from a previous natural infection. This study would involve you taking a slightly larger blood sample that would be sent to a laboratory for testing.
Information inserted on potential further blood sample collection to determine whether positive antibody test results are due to natural infection or COVID-19 vaccination.
All UK Biobank participants, including those who have already received one or more doses of a COVID-19 vaccination, are eligible to take part. We will ask you whether you have received a vaccine, and the date of your vaccination (if applicable) when you send us your test result.
If you would like to take part, we will ask you to confirm that we have your correct address so that we can send you the testing kit, and to provide consent to take part. If you are willing to do so, please provide us with a mobile phone number so that we can confirm receipt of your test result. To join the study, please log into the UK Biobank participant website, where you can:
- check your contact details; and
- complete our online consent form.
If you do not want to take part, we would be grateful if you could let us know by logging into the UK Biobank participant website.
The study is only open to UK Biobank participants who will be invited over two phases of the study. If your partner/spouse is a participant in UK Biobank and has not yet received an invitation, they should wait to receive one. They can also log into the participant website to see if we are ready for them to take part. Here they can check their contact details and consent to participate by clicking on the “Antibody Study” button (which will only be displayed if we are ready for them to take part).
If you agree to take part, we will send you the antibody self-testing kit within two weeks of receiving your consent. We will email you to let you know that your home antibody testing kit is on its way.
If you do not have any symptoms of COVID-19, please take the test as soon as possible after you receive your kit. We will send you a reminder if we have not received a result from you 7-14 days after sending you your kit.
If you do have symptoms of COVID-19 when you receive your kit, please do not use the test until at least 14 days after the onset of symptoms. If this is the case, please ignore our reminder and take the test as soon as you are able to – there is no need to tell us that your result will be delayed.
The main symptoms of COVID-19 are:
- A high temperature
- A new continuous cough
- A loss or change to sense of smell or taste.
The kit will contain full instructions about how to carry out the test safely and easily. These instructions can also be found on our website, along with a video that shows you how to take the test.
If you consent to join the study and then decide that you no longer wish to carry out the test, you do not need to do anything. Please dispose of the test kit in your usual household waste.
After 10 minutes of doing the test, the viewing window will show your result. It will show one of the following combinations of lines (the colour intensity may vary). The position of the lines will show if your test result is positive or negative.
If there is a problem with your kit that prevents you from taking the test, a replacement kit can be requested by logging into the UK Biobank Participant website at https://biobank.ndph.ox.ac.uk/members/ and clicking on “Replacement kit”. Here you can provide details about any problems encountered with your antibody testing kit.
If you have an adverse reaction, are harmed as a result of undertaking the test, or had any other problems that did not require you to request a replacement kit, please contact our Participant Resource Centre on 0800 0 276 276.
We would like you to tell us the result of your test (i.e. whether it is positive, negative or invalid).
When you are ready to give UK Biobank your test result, please log into the UK Biobank participant website and click on “Enter test result” where you can provide us with your result via the “test result” questionnaire.
Please dispose of your used test kit with your regular household waste, as directed in the kit information sheet.
The test result and accompanying data will be incorporated into the UK Biobank resource and made available (in a de-identified form so that you cannot be identified) to approved researchers for vital COVID-19 research.
Yes. The antibody test will provide you with a result in 10 minutes, without the need to send a sample to a laboratory. The tests carried out as part of this study are for research purposes only. The result of your home antibody test should not be used to confirm or eliminate whether you have been infected or whether you have immunity against the coronavirus.
We also do not currently know if these antibodies will protect you from getting COVID-19. Therefore, whatever your test result, you should continue to follow government legislation and guidelines. Please do not make any medical or personal safety decisions based on the result of this test.
You will receive information about whether you have antibodies to the coronavirus (and therefore whether it is likely that you have been previously infected), which may be of interest to you.
Your involvement will enable important research to take place to understand the extent of coronavirus infection and enable researchers to determine the risk factors for COVID-19 and its long-term health effects. This, in turn, may help scientists to find new ways to manage the outbreak and to prevent or treat COVID-19.
Participation in the study involves doing a home antibody self-test by obtaining a drop of blood from a finger-prick. The finger-prick should be done on the ring or middle finger on your non-dominant hand using a device called a lancet. This produces a small (less than 1mm) prick in the skin.
You may experience slight discomfort when using the lancet (people have described the feeling as a slight sting, much less than you would experience in a typical blood collection with a needle and syringe) and, as with any cut, there is a small risk of infection and/or bruising. However, the equipment is sterile and following the instructions provided in the kit will minimise risk of infection (such as cleaning the area before and after).
The process and products in the kit are used routinely in a wide range of healthcare settings, including for home measurement of blood glucose levels in the management of diabetes.
Yes, this study has received ethics approval from the North West – Haydock Research Ethics Committee, 16/NW/0274.
The information obtained by completing this test will be treated in exactly the same way as the other data we have collected about you. The data will be kept by UK Biobank for many years. They will be provided in a de-identified form (such that no participant can be identified from their data or samples) to approved researchers for medical and other health-related research (including scientists working in other countries and in commercial companies).
We will put the results from all of these studies back into our database for other researchers to use. Scientists must make public the results of all research based on the resource so that everyone can benefit from it. You can find details of research that is being done using the UK Biobank resource and the related publications on our website (www.ukbiobank.ac.uk).
We have strict measures in place to protect your confidentiality. These include the following:
- We do not include any details that will identify you in any information or samples we provide to researchers.
- We keep any information that might identify you (such as your name and address) separately from other information about you in our database.
- We use advanced computer security technologies to prevent unauthorised access to the computers that hold personal information.
- Our procedures comply with international standards (ISO/IEC 27001) for information security and management. We are externally audited and we comply with the guidance contained in the UK Government’s ten cyber security steps.
- We restrict access to personal information as much as possible, and all research staff working for us sign confidentiality agreements as part of their employment contracts.
This should prevent identifiable information from being used – inadvertently or deliberately – for any purpose other than to support this study.
The basis on which we process your personal data, which will include any personal data collected for the purpose of the UK Biobank coronavirus self-test antibody study, is explained on our website at https://www.ukbiobank.ac.uk/explore-your-participation/basis-of-your-participation. This includes an explanation of the way in which we protect your data and remove any personal identifiers before making any data available to researchers.
If you would like further information or have any concerns about anything to do with UK Biobank, please refer to our frequently asked questions, telephone our Participant Resource Centre free of charge on 0800 0 276 276 (Monday to Friday 9am to 5pm), or email us at ukbiobank@ukbiobank.ac.uk
Alternatively, if you would like to write to the person in charge, please send your letter to:
Professor Sir Rory Collins
Principal Investigator, UK Biobank
1-2 Spectrum Way
Adswood
Stockport
Cheshire
SK3 0SA
We shall reply to your letter in writing, unless you enclose your telephone number so that we can call you to discuss your concerns.
UK Biobank Limited (company no. 4978912) is a registered charity in England & Wales (1101332) and in Scotland (SC039230)
IRAS project ID 200778
Version 2.1 02/03/2021
Last updated