Frequently Asked Questions: Phase 2
The phase 2 study uses the AbC-19TM Rapid test kit
Frequently Asked Questions: Phase 2 (AbC-19 Rapid Test)
This section of the website provides you with more detailed information on all aspects of phase 2 of the coronavirus antibody self-test study in which you have been invited to participate, including: how to take part; details of the AbC-19 Rapid antibody self-test; delivery of your test kit; taking the test; and sending UK Biobank your result. Please click on the arrow next to each question to reveal the relevant answer.
I have been vaccinated but my test result is negative. Why is that?
Some people who have been vaccinated will get a ‘negative’ result on the antibody test. This may happen for a number of reasons and does not mean that your vaccination has not worked. Please note that we do not intend to send you a second test kit if you have been vaccinated or obtain a negative result, as the purpose of the study is to determine whether you are likely to have been previously infected with SARS-CoV-2, rather than to assess antibody status following vaccination. Do not make any medical or personal safety decisions based on the result of this test, which is being done for research purposes only. Continue to follow government COVID-19 legislation and guidelines, and do not change your behaviour based on your test result.
Find out more below.
Professor Naomi Allen, Chief Scientist at UK Biobank, explaining why a negative result following an antibody test does not mean your vaccine has not worked.
- Studies have shown that vaccines protect you from developing serious symptoms of COVID-19 within 1-2 weeks of the first dose, even though antibodies take much longer to develop. Consequently, it may take some time for your blood antibodies to reach a high enough level to be detected by the antibody test, even after you have had the second dose of the vaccine. This does not mean they are not present, just that they are not high enough to be above the threshold for detection by the kit.
- Factors such as age or particular medicines that you may be taking (such as drugs that suppress the immune system) may determine how quickly your body produces antibodies following vaccination. For example, those over the age of 70 take longer to develop antibodies compared to younger individuals.
- It is important to note that the immune response to the vaccine is complex and does not only depend on antibodies. Vaccines also induce a T cell response (one of the important white blood cells of the immune system), which our tests do not measure.
Other common questions
We would still like you to complete the test if you have received a coronavirus vaccine; we will ask you about this when you tell us your result.
No. Once you have completed your test, you must read the test result immediately after 20 minutes and no longer than 30 minutes after. After this time, the result will no longer be accurate and should be ignored. You do not need to take another test.
Our Participant Resource Centre is currently experiencing very high call volumes. If you can, please submit your test result by logging into the UK Biobank participant website at www.ukbiobank.ac.uk/members, clicking on “Enter test result” and completing the test result questionnaire.
Taking part
UK Biobank is a large biomedical database and research resource that contains the genetic, lifestyle and health information from half a million UK participants, of which you are one.
Volunteers who were aged between 40-69 years when recruited between 2006 and 2010 provided detailed information about lifestyle, as well as having physical measures taken and blood, urine and saliva samples collected and stored for future analysis.
The UK Biobank resource, which now also includes genetic and electronic health records for all participants, and imaging scans from around 50,000 of the participants, is globally accessible to approved researchers doing health-related research that is in the public interest.
UK Biobank’s research resource is a major contributor to the advancement of modern medicine and treatment, enabling better understanding of the prevention, diagnosis and treatment of a wide range of serious illnesses – including cancer, heart diseases, stroke and now COVID-19.
While taking part in UK Biobank is not intended to help participants directly, it should give future generations a better chance of living their lives free of diseases that disable and kill.
UK Biobank is supported by its founding funders, the Wellcome Trust and UK Medical Research Council, as well as the Department of Health, Scottish Government, the Northwest Regional Development Agency, British Heart Foundation and Cancer Research UK. The organisation has over 150 dedicated members of staff, based in multiple locations across the UK.
No, participation in this study is entirely voluntary. However, every person that takes part will help us to collect vital information for COVID-19 research.
Yes. We are interested in antibody levels in people who have had the vaccine as well as those who have not. We will ask you about this when you tell us your test result.
Yes. We will ask if you have had a vaccine when you tell us your test result. Please do not delay having your vaccine because you are taking part in this study.
If you do not want to take part, we would be grateful if you could let us know by logging into the UK Biobank participant website using your personal details and PID (this can be found on your invitation email/letter), clicking on the " Antibody Study" button and indicating that you do not wish to participate.
Yes. This study has different aims and objectives to the monthly serology study and we would like as many people to participate in this study as possible.
No. This study is only open to UK Biobank participants who attended an assessment centre 10-15 years ago.
Yes. Taking part in another study will not affect your ability to take part in the UK Biobank antibody self-test study.
If you have been unblinded (i.e. you know whether you received the vaccine or a placebo), you can take part. If you do not know this, please wait until you have been unblinded before you consent to participate.
The purpose of this study is to determine the extent to which the UK Biobank study population has been previously exposed to the SARS-CoV-2 coronavirus. It is therefore important to include as many people as possible, regardless of whether you have experienced any COVID-19 symptoms.
If you have symptoms of COVID-19 when you receive your kit, please do not take the test until at least 14 days after the onset of symptoms.
The main symptoms of COVID-19 are:
- A high temperature
- A new continuous cough
- A loss or change to sense of smell or taste.
Yes. We want to include as many UK Biobank participants as possible. The test is not suitable for use by anyone who has an untreated blood coagulation disorder. However, if you are taking anti-coagulant medication, the amount of blood that needs to be collected is very small (a 2-3mm sized spot) and provides no additional risk than any other finger-prick test so it should not affect you unduly.”
Yes. We want to include as many UK Biobank participants as possible. The test is not suitable for use by anyone who has an untreated blood coagulation disorder. However, if you are taking anti-coagulant medication, the amount of blood that needs to be collected is very small (a 2-3mm sized spot) and provides no additional risk than any other finger-prick test so it should not affect you unduly
The study is only open to UK Biobank participants. Other household members, relatives and friends who are not current UK Biobank participants cannot take part. If you register to take part and receive a test, this must not be completed by anyone else. If you do not want to complete the test after receiving it, please dispose of it safely and do not pass it on to anyone else.
To dispose of the test kit, please:
- Place the testing stick back into its foil wrapper.
- Press and click any unused lancets.
- Place all of the test kit items into the waste bag.
- Dispose of the kit with your regular household waste.
- Keep out of reach of children and pets.
For the study findings to be useful, it is important that the test is only undertaken by UK Biobank participants so that we can incorporate the results with the existing data that we hold for research purposes.
If your spouse/partner is a participant in UK Biobank and has not yet received an invitation to participate, they should wait to receive on. They can also log in to the UK Biobank participant website at www.ukbiobank.ac.uk/members/ to see if we are ready for them to take part. Here they can check their contact details and consent to participate by clicking on the "Antibody Study" button (which will only be displayed if we are ready for them to take part).
No. In order for the information we gather during this study to be useful to researchers, it is important that everyone involved uses the antibody testing kit that we send them.
Yes.
Yes.
Please register your interest by logging into the UK Biobank participant website at www.ukbiobank.ac.uk/members/ . Contact details can be checked and updated by clicking on the “Contact Details” button on the participant homepage. To consent to take a test for this research, please click on the “Antibody Study” button on the participant homepage.
Alternatively, if you were invited by post and still have the consent form you were sent, you can return this to us in the prepaid envelope.
If you have any other issues registering to take part, please call our Participant Resource Centre on 0800 0 276 276 (Monday to Friday 9am to 5pm).
If you have provided us with an email address, you should receive an acknowledgement email within one working day. Please check your junk/spam folder as well as your inbox. If we do not have an email address for you, you should receive your kit within two weeks of returning your consent form.
Once your kit has been despatched, you can view your progress in this study (including whether we have sent your kit or received your test result) by logging into the UK Biobank participant website at www.ukbiobank.ac.uk/members/ and clicking on “View study progress”.
If you have already received your first kit, please dispose of it in your general waste following the instructions in the kit. If you have received a second kit and you no longer wish to take part, please call our Participant Resource Centre on 0800 0 276 276 (Monday to Friday 9am to 5pm) then dispose of the kit in your general waste following the instructions in the kit. For the study findings to be useful, and so that we can incorporate the findings into the UKB resource, it is important that your test is not completed by anyone else
The antibody self-test
The AbC-19 Rapid TM Test is a single-use test for the detection of SARS-CoV-2 IgG antibodies in capillary blood. The test requires a small drop of blood from a finger-prick and shows results in 20 minutes, without the need to send a sample to a laboratory.
The kits were donated to UK Biobank by the Department of Health and Social Care.
In order to start the study as quickly as possible, we used the Fortress Fast COVID-19 Device in phase 1 of the study (which took place February-March) as this device has been used in other research studies and we were able to obtain rapid regulatory approval for its use in UK Biobank. The AbC-19 Rapid TM kit being used in phase 2 (the current phase of the study) took longer to obtain the necessary approvals for use. Both kits can detect whether you have antibodies to the SARS-CoV-2 virus.
Yes. You will be able to see the outcome of the antibody test within 20 minutes. We would like you to send us the result of your test as soon as possible after you have done it. As with all antibody tests, the results are not 100% accurate and should not be used to guide your behaviour. A positive test does not necessarily mean you have had COVID-19 and it is important that you continue to follow the current government COVID-19 legislation and guidance. If you are worried about currently having COVID-19, you should follow the current government/NHS guidance about obtaining a diagnostic test or call 111.
The AbC-19TM Rapid Test detects IgG antibodies to the spike protein of the SARS-CoV-2 virus. The device has been developed by Abingdon Health and is CE marked for sale for professional use and registered with the Medical and Healthcare products Regulatory Agency (MHRA). UK Biobank’s use the AbC-19TM Rapid Test as a self-test was reviewed by the MHRA in March 2021.
There are certain circumstances in which a false negative result may occur (i.e. where the test does not identify antibodies even though you have been infected previously with the virus), including if the test is performed less than 14 days after the first signs of infection.
Age, pre-existing illnesses that cause the immune system to be weakened, or the use of medications such as immunosuppressants (which, for example, are often used for the treatment of arthritis) may reduce the speed at which your body produces antibodies following infection. As a result, the level of antibodies to the SARS-CoV-2 virus in your blood may be below the detection limit of the test.
Consequently, the results should not be used to determine whether you have been infected or whether you have immunity against the coronavirus. Please do not use the test result to guide your behaviour; it is important that you continue to follow the current government legislation and guidelines.
Upon completion of the test, you will receive the result of the antibody test. However, as with all antibody tests, the results are not 100% accurate and should not be used to guide your behaviour; it is important that you continue to follow the current government legislation and guidelines. There will be no monetary benefit as a result of taking part in this study, but your involvement will enable important research to take place to understand the extent of coronavirus infection and enable researchers to determine the risk factors for COVID-19 and its long-term health effects. This, in turn, may help scientists to find new ways to manage the outbreak and to prevent or treat COVID-19.
If you test positive and you have not yet received a COVID-19 vaccination, we would like you to take a second test (that we will send to you when you tell us your first result). This is to make sure that your test result is truly positive. This is important as we want to reduce the number of false positives as much as possible (i.e. those who report as positive but are actually negative).
Delivery of my test kit
If you agree to take part, we will send you a home self-test kit within two weeks. If we have an email address for you, we will send you an email a few days before your antibody test kit is due to arrive. If you have not received your kit within 14 days of your email notification, you can request another one by logging into the UK Biobank participant website at www.ukbiobank.ac.uk/members/ and clicking on “Replacement kit”.
If you were invited to join the study by post and agreed to participate by posting back the consent form to us, please wait for 28 days after registering to take part before you contact us via the UK Biobank website (as described above) to request a replacement if your kit has not arrived.
Your test kit will be delivered to your home by Amazon Shipping or Royal Mail.
These organisations meet UK Biobank’s standards for the handling of personal data. Further information can be obtained from https://ship.amazon.co.uk/privacy and https://www.royalmail.com/business/privacy-policy/.
Please log into the UK Biobank participant website at https://biobank.ndph.ox.ac.uk/members/ and click on the “Contact Details” button to update your details. Alternatively, if you were invited to participate via post, you can include your change of details on the completed consent form.
The kit should be delivered to a secure letter/parcel box where possible. Unfortunately, we are unable to take specific delivery instructions.
Yes.
If you intend to return to your home address within the next two weeks then there is no need for us to send the kit elsewhere; simply complete the test as soon as possible on your return home. You do not need to tell us about this.
If you do not intend to return to your home address in the next two weeks then please provide us with an alternative UK postal address via our participant website prior to consenting to participate. If you do this, please remember to update your postal address when you return home. If your kit has already been despatched to your home address, please log into the UK Biobank participant website at https://biobank.ndph.ox.ac.uk/members/ , update your contact details with the address to which your kit should be sent, and click on “Replacement kit” to request a replacement kit be sent to this temporary address.
No. If you are unable to take your test when the kit is sent to you, please perform the test at your earliest convenience.
Taking my test
If you are not currently experiencing any symptoms of COVID-19, please perform the test and provide us with the result as soon as you can. You can take the test at any time of the day.
If you have symptoms of COVID-19 when you receive your kit, do not use the test until at least 14 days after the onset of symptoms.
The main symptoms of COVID-19 are:
- A high temperature
- A new continuous cough
- A loss or change to sense of smell or taste.
No, but we would be grateful if you would complete it as soon as you can. We will send you an email reminder if you have not returned your test result within seven days of receiving your test kit. If we do not have an email address for you but do have a mobile number for you we will send you a text message reminder if you have not returned your test result within fourteen days of receiving your test kit.
No.
Yes, please visit https://ukbiobank.ac.uk/antibody-sample to view a copy of the instructions for use and a video showing how to take your test.
Yes. If any components are missing from the kit or if any of the pouches have been opened, please do not use the test. Please log into the UK Biobank participant website at www.ukbiobank.ac.uk/members/ and click on “Replacement kit” to request a replacement kit and provide us with details of the problem. Unfortunately, you are unable to request a replacement kit if you have already provided us with your test result.
Please take your test and send us the result as soon as you can to help avoid parts becoming lost or damaged. If you do need a new kit, please log into the UK Biobank participant website at www.ukbiobank.ac.uk/members/ and click on “Replacement kit” to request a replacement kit and provide us with details of the problem. Unfortunately, you are unable to request a replacement kit if you have already provided us with your test result.
If there is a problem with your kit that prevents you from taking the test, a replacement kit can be requested by logging into the UK Biobank website at www.ukbiobank.ox.ac.uk/members/ and clicking on “Replacement kit”. Here you can provide details about any problems encountered with your antibody testing kit. Unfortunately, you are unable to request a replacement kit if you have already provided us with your test result.
If you had an adverse reaction, were harmed as a result of undertaking the test, or had any other problems that did not require you to request a new kit, please contact our Participant Resource Centre on 0800 0 276 276.
The test involves pricking the tip of your finger to get a single spot of blood for testing. All of the instructions will be provided with the kit.
The blood collection pipette should be filled to the black line marked on the side of the collector.
No; the test has been validated based on the use of a specific and very small amount (5µl) of whole blood which is indicated by the black line on the blood collection pipette. Failure to follow the steps indicated in the instructions for use could lead to inaccurate results being obtained. The blood collection pipette cannot be re-used so it is important that you do not overfill or underfill it. If you are struggling, we recommend asking someone to help you if possible.
The test only requires approximately 3-4 drops of test solution. As detailed in the instructions, apply the test solution to the sample hole, one drop at a time, until there is no test solution remaining. Due to the small amount of test solution required, you may experience some bubbling when applying the solution to the test.
Once you have read the instructions, it should take no longer than a few minutes to take the test. Once you have done the test, you will be able to see the result within 20 minutes.
Please refer to the instructions that accompanied the test kit for full details of how to take the test. Someone else in your household may also be able to help if you are having trouble. If you require any further information about doing the test, it may help to visit www.ukbiobank.ac.uk/antibody-sample to watch a video showing the collection process. You can also call our Participant Resource Centre on 0800 0 276 276 (Monday to Friday 9am to 5pm).
We do not recommend collecting blood from any other part of your body as the instructions are written to optimise blood collection from your finger. Please read the instructions carefully to maximise the chance of taking a finger prick amount of blood as easily as possible.
When using the lancet, you will feel a small sharp scratch. As the lancet is retractable, no needle will be visible at any point. For further guidance on how the test is performed, please watch the instruction video here.
No. Unless you are assisting another participant to complete the test, no personal protective equipment is necessary.
No, do not attempt to reuse the test. It is a single-use disposable device which is not intended for multiple uses. The device will not register a second test and reusing a single-use lancet may risk infection. Please dispose of the kit securely once you have completed your test.
- Place the testing stick back into its foil wrapper.
- Press and click any unused lancets.
- Place all of the test kit items into the waste bag.
- Dispose of the kit with your regular household waste.
- Keep out of reach of children and pets.
Yes. Please log into the UK Biobank participant website at www.ukbiobank.ac.uk/members/ and click on “Replacement kit” to request a replacement kit and provide us with details of the problem. Unfortunately, you are unable to request a replacement kit if you have already provided us with your test result.
Once the test has been performed, up to two lines can appear on the test. The line furthest away from the sample hole is the control line (C-line). The C-line is always present if the test has been performed correctly. If the C-line is present then the test has worked.
Instructions about how to read your test are included in the leaflet sent with your test kit.
After 20 minutes of taking the test, the viewing window will show your result. Your test will show one of the following combinations of red lines (the colour intensity may vary). The position of the red lines will show if your test result is positive or negative.
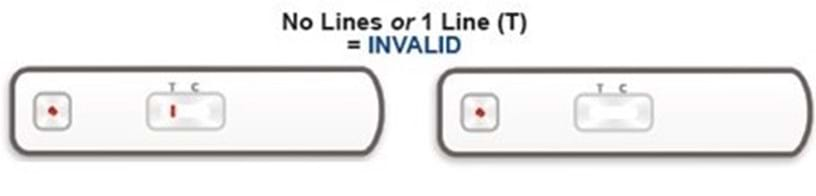
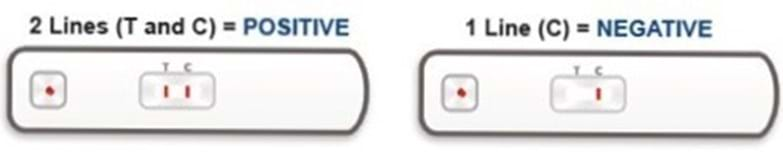
The red line closest to the sample hole is the test line (T-line). The presence of a T-line alongside a C-line is a POSITIVE result.
We do not currently know if antibodies present in your blood will protect you from getting COVID-19 again. Therefore, whatever your test result, you should continue to follow government legislation and guidelines. Please do not make any medical or personal safety decisions based on the result of this test. For the current government guidance about COVID-19, please visit https://www.gov.uk/coronavirus.
Yes; provided you have a C-line, a visible T-line of any intensity should be interpreted as a positive result.
The test should be read immediately after 20 minutes following the addition of the sample and test solution and no longer than 30 minutes after. Reading the results after this time could lead to inaccurate results.
The test result
A positive test result indicates that you have probably been previously infected by the coronavirus that causes COVID-19 and have developed antibodies against the virus. However, a positive test may also be a consequence of a recently received vaccine. If you have received a single dose of your COVID-19 vaccine and obtain a ‘positive’ result, it is still very important to obtain the second dose of your vaccine.
If you obtain a positive test and you have not yet received a COVID-19 vaccination, we will send you a second kit to double-check the result. We will do this within a couple of weeks of receiving your first test result from you.
We do not yet know if having blood antibodies will protect you from getting COVID-19 again. The result of the test also does not tell us anything about infectivity i.e. whether you have an active infection that can be passed on to others. Therefore, whatever your test result, you should continue to follow current government legislation and guidelines regarding social distancing and self-isolation. If you have received a single dose of your COVID-19 vaccine and obtain a ‘positive’ result on your antibody test, it is still very important to obtain the second dose of your vaccine.
If you test positive, you may be invited to take part in a further study which will allow us to see if the antibodies are a result of your vaccination or from a previous natural infection. This study would involve you taking a slightly larger blood sample that would be sent to a laboratory for testing.
A positive test result does not necessarily mean you are immune to developing COVID-19 in the future. Therefore, whatever your test result, you should continue to follow current government legislation and guidelines. If you have received a single dose of your COVID-19 vaccine and obtain a ‘positive’ result on your antibody test, it is still very important to obtain the second dose of your vaccine.
The result of this test does not tell you anything about infectivity (i.e. whether you have the active infection and can transmit it to others). It only tells you whether you have likely been infected with the virus at some time in the past. You should continue to follow the current government guidance regarding social distancing and self-isolation, regardless of the result of this test.
Yes, you must continue to follow current government guidance on social distancing.
Not because this test result is positive. However, if you have symptoms that suggest you may have COVID-19, you and your household must follow self-isolation guidelines.
If your test result is negative, this means the test has not found antibodies to the SARS-CoV-2 coronavirus in your blood. There is still a lot to learn about this virus and it should be noted that if antibodies are not present in your blood, it does not necessarily mean that you have not been infected with the virus. You should continue to follow current government legislation and guidance.
Some people who have been vaccinated will get a ‘negative’ result on the antibody test. This may happen for a number of reasons and does not mean that your vaccination has not worked.
Studies have shown that vaccines protect you from developing serious symptoms of COVID-19 within 1-2 weeks of the first dose, even though antibodies take much longer to develop. Consequently, it may take some time for your blood antibodies to reach a high enough level to be detected by the antibody test, even after you have had the second dose of the vaccine. This does not mean they are not present, just that they are not high enough to be above the threshold for detection by the kit.
Factors such as age or particular medicines that you may be taking (such as drugs that suppress the immune system) may determine how quickly your body produces antibodies following vaccination. For example, those over the age of 70 take longer to develop antibodies compared to younger individuals.
It is important to note that the immune response to the vaccine is complex and does not only depend on antibodies. Vaccines also induce a T cell response (one of the important white blood cells of the immune system), which our tests do not measure. To hear UK Biobank’s Chief Scientist Professor Naomi Allen explaining this in more detail, please go back to the top of this webpage and click on the video.
We do not intend to send you a second test kit if you have been vaccinated and obtain a negative result, as the purpose of the study is to determine whether you are likely to have been previously infected with SARS-CoV-2 rather than to assess antibody status following vaccination.
Following infection, it may take some people a long time to generate antibodies to levels that are detectable by the test (and some may never generate a sufficiently strong response). For example, individuals who are taking certain medications, such as those that suppress the immune system, or elderly individuals - particularly those over the age of 70 - may only generate a weak antibody response. This does not mean that antibodies are not present, just that they are not at a high enough level to be detected by the kit.
If you have previously tested positive for SARS-CoV-2 with a lateral flow test (either an antigen kit that measures for active infection or an antibody test that measures for past infection), it is possible that the previous test provided a false positive result. This is the reason we are sending a second antibody kit to those who test positive to double-check the result (in order to reduce the number of false-positives).
The reason we send you a second kit if you test positive for SARS-CoV-2 antibodies and have not yet had the vaccine is because we know that there can be ‘false-positives’, whereby you are incorrectly assigned as having antibodies when, in fact, you do not. No test is 100% accurate and in order to reduce the number of false-positives, we would like to double-check the result by sending you a second kit. Hence, if you tested positive on your first antibody test but negative on your second test, it is possible that your first result was a ‘false positive’ result.
If your test result is invalid, this means the test has not worked and it is not possible to give you a result. The test needs to be repeated using a new kit and a fresh blood sample. If you would like us to send you a new kit, please log into the UK Biobank participant website at www.ukbiobank.ac.uk/members and click on “Replacement kit” to request a replacement kit and provide us with details of the problem. Unfortunately, you are unable to request a replacement kit if you have already provided us with your test result.
Sending UK Biobank my information
When you are ready to send UK Biobank your test result, please log into the UK Biobank participant website at https://biobank.ndph.ox.ac.uk/members/ and click on “Enter test result” to complete the online “test result questionnaire”.
If you experience difficulties completing the test result questionnaire, please contact our Participant Resource Centre on 0800 0 276 276.
Please let us know by emailing us at ukbiobank@ukbiobank.ac.uk or by telephoning us on 0800 0276 276 so that we can update our records.
The test results and questionnaire data you provide us with will be incorporated into the UK Biobank resource and made available to approved researchers for vital COVID-19 research.
After you have reported it to us, your test result and related information will be kept by UK Biobank for many years. They will be provided in a de-identified form (such that no participant can be identified from their data or test result) to approved researchers for medical and other health-related research (including scientists working in other countries and in commercial companies).
We keep any information that might identify you (such as your name and address) separately from other information about you in our database.
The basis on which we process your personal data, which will include any personal data collected for the purpose of the UK Biobank coronavirus self-test antibody study, is explained on our website at https://www.ukbiobank.ac.uk/explore-your-participation/basis-of-your-participation. This includes an explanation of the way in which we protect your data and remove any personal identifiers before making any data available to researchers.
Last updated
