Information sheet: Phase 2
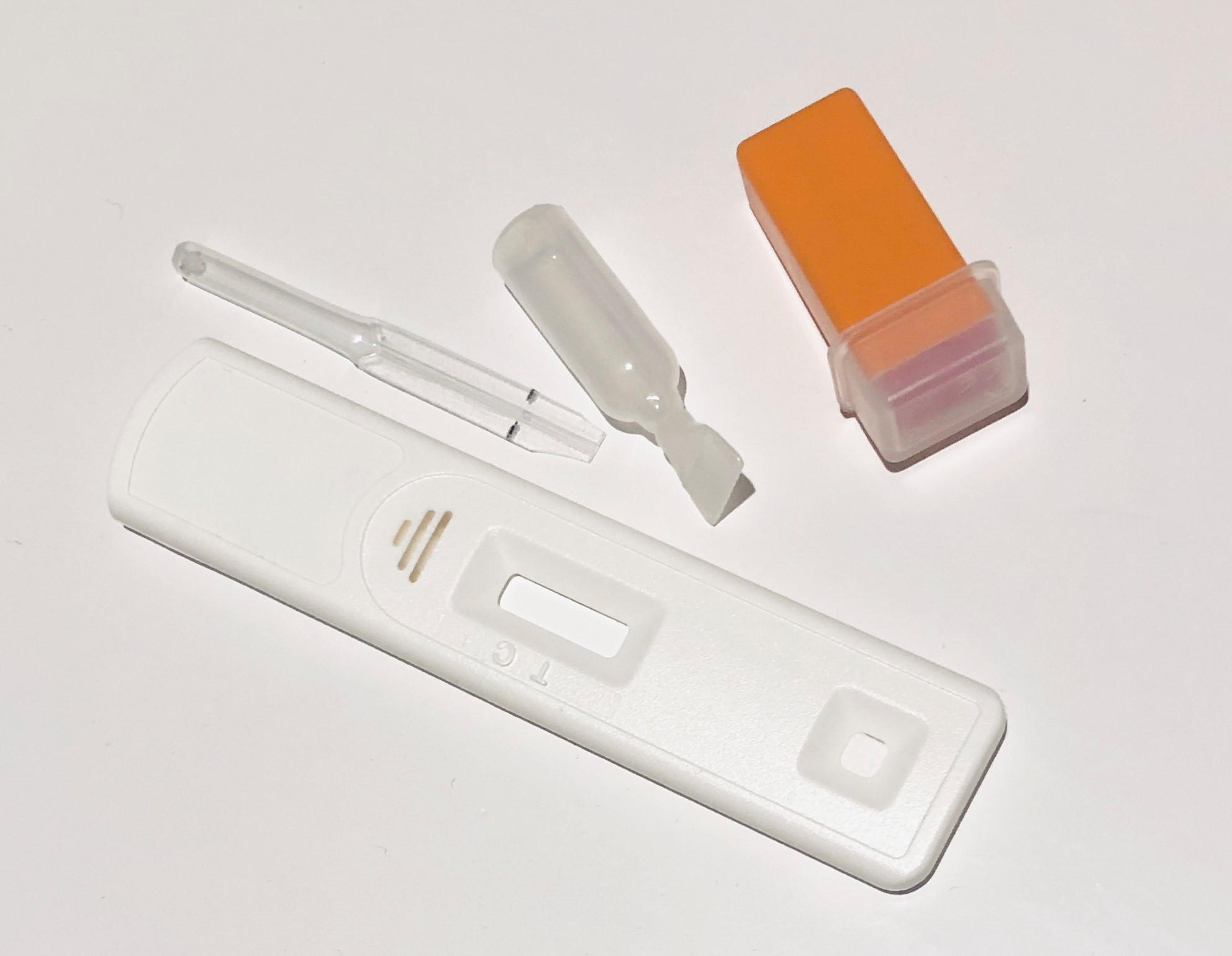
The phase 2 study uses the AbC-19TM Rapid test kit
Information sheet: Phase 2 (AbC-19TM Rapid Test)
We are inviting all UK Biobank participants to help UK Biobank undertake urgent research on the SARS-CoV-2 coronavirus that causes COVID-19. This new study will provide researchers with unique opportunities to investigate the long-term health effects of SARS-CoV-2 infection (“Long COVID”) and other health research related to COVID-19.
The study involves doing a simple antibody test (the AbC-19TM Rapid Test) at home, which will tell you if it is likely that you have previously been infected with the coronavirus. If you agree to help, we will send you a finger-prick self-testing kit to find out if you have antibodies to the coronavirus. Taking the test is simple and quick to do and you can let us know the result via the UK Biobank participant website.
Taking part is entirely voluntary and will not affect your ongoing relationship with UK Biobank. Please take the time to read this information carefully. It explains why we are asking you to help and what it would involve.
If anything is not clear, or if you would like more information, please refer to our frequently asked questions, You can find more information here.
If you decide that you would like to take part after having read all of the information below, please log on to our participant homepage, check your contact details, then click on “Antibody Study” to give your consent to take part in the study.
Thank you for your continued support
This research study aims to measure the extent of SARS-CoV-2 (coronavirus) infection in the UK Biobank population by measuring blood antibodies to the virus. This information will enable researchers to determine the genetic and clinical risk factors for COVID-19, explore factors which influence COVID-19 disease severity and understand the long-term health effects of SARS-CoV-2 infection (“Long COVID”). This research may, in turn, lead to better ways to manage the disease.
We are inviting all UK Biobank participants to take part.
Please note that this study is different from UK Biobank’s coronavirus study involving the collection of repeated blood samples for 6 months. Even if you took part in that study, we would still like you to take part in this new study.
No; whilst we are keen for all of our participants to contribute, your participation is entirely voluntary. We do understand that you may not be able or wish to help and this will not affect your future relationship with UK Biobank.
We will ask you to:
- Use a home antibody self-testing kit (ABC-19TM Rapid Test), which we will deliver to your home. This kit uses a small drop of blood from a finger-prick to measure the presence of blood antibodies to the SARS-CoV-2 virus. It will provide you with a result in 20 minutes.
- Log into the UK Biobank participant website to let us know the result of the test and whether you have had a COVID-19 vaccination.
- If your test indicated that you have the antibodies (a ‘positive’ result) and you also tell us that you have not been vaccinated, we will ask you to repeat the test to double-check the result. We will send you a second kit within a couple of weeks of receiving your first test result.
All UK Biobank participants, including those who have already received one or more doses of a COVID-19 vaccine, are eligible to take part. We will ask you whether you have received a vaccination and the date of your vaccination (if applicable) when you send us your test result.
If you would like to take part, we will ask you to confirm that we have your correct address so that we can send you the testing kit, and to provide consent to take part. If you are willing to do so, please provide us with a mobile phone number so that we can confirm receipt of your test result. If you do not have an email address or mobile phone number, you will still be able to participate.
To join the study, please log into the UK Biobank participant website, where you can:
- check your contact details; and
- complete our online consent form.
If you do not want to take part, we would be grateful if you could let us know by logging into the UK Biobank participant website, clicking on the "Antibody Study" button and indicating that you do not wish to participate.
The study is only open to UK Biobank participants, who will be invited over two phases of the study. If your partner/spouse is a participant in UK Biobank and has not yet received an invitation, they should wait to receive one. They can also log into the participant website to see if we are ready for them to take part. Here they can check their contact details and consent to participate by clicking on the “Antibody Study” button (which will only be displayed if we are ready for them to take part).
We will send you the antibody self-testing kit within two weeks of receiving your consent. We will email you to let you know that your home antibody testing kit is on its way (if we have an email address for you).
If you do not have any symptoms of COVID-19, please take the test as soon as possible after you receive your kit. We will send you a reminder if we have not received a result from you 7-14 days after sending you your kit.
If you do have symptoms of COVID-19 when you receive your kit, please do not use the test until at least 14 days after the onset of symptoms. If this is the case, please ignore our reminder and take the test as soon as you are able to – there is no need to tell us that your result will be delayed. The main symptoms of COVID-19 are:
- A high temperature
- A new continuous cough
- A loss or change to sense of smell or taste.
The kit will contain full instructions about how to carry out the test safely and easily. These instructions can also be found on our website, along with a video that shows you how to take the test.
If you consent to take part and then decide that you do not wish to carry out the test, you do not need to do anything. Dispose of the test kit in your usual household waste.
For the study findings to be useful, and so that we can incorporate the findings into the UK Biobank resource, it is important that your test is not completed by anyone else.
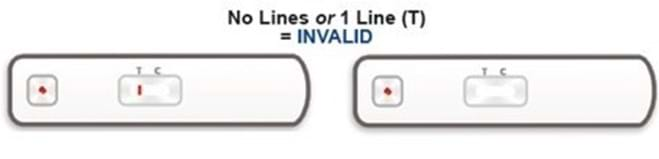
After 20 minutes of doing the test, the viewing window will show your result. It will show one of the following combinations of red lines (the colour intensity may vary). The position of the red lines will show if your test result is positive, negative or invalid.

If there is a problem with your kit that prevents you from taking the test, a replacement kit can be requested by logging into the UK Biobank participant website, clicking on “Replacement kit” and providing details about any problems encountered with your antibody testing kit. Unfortunately, you are unable to request a replacement kit if you have already provided us with your test result.
If you have an adverse reaction, are harmed as a result of undertaking the test, or had any other problems that did not require you to request a replacement kit, please contact our Participant Resource Centre on 0800 0 276 276.
We would like you to tell us the result of your test (i.e. whether it is positive, negative or invalid).
When you are ready to give UK Biobank your test result, please log into the UK Biobank participant website and click on “Enter test result” to provide us with your result via the test result questionnaire. If you are unable to do this online and were invited to join the study via post, please complete the paper test result questionnaire and return it to us in the pre-paid envelope provided with your test kit.
Please dispose of your used test kit with your regular household waste, as directed in the kit instruction booklet.
If you test positive and you have not yet received a COVID-19 vaccination, we would like you to take a second test (that we will send to you when you tell us your first result). This is to make sure that your test result is truly positive. This is important as we want to reduce the number of false positives as much as possible (i.e. those who report as positive but are actually negative).
If you test positive and you have received a COVID-19 vaccination, you may be invited to take part in a further study which will allow us to see if the antibodies are a result of your vaccination or from a previous natural infection. This study would involve you taking a slightly larger blood sample that would be sent to a laboratory for testing.
The test result and accompanying data will be incorporated into the UK Biobank resource and made available (in a de-identified form so that you cannot be identified) to approved researchers for vital COVID-19 research.
Yes. The antibody test will provide you with a result in 20 minutes, without the need to send a sample to a laboratory.
The tests carried out as part of this study are for research purposes only. The result of your home antibody test should not be used to confirm or eliminate whether you have been infected or whether you have immunity against the coronavirus after vaccination. This is because false negative results may be observed in certain circumstances, including if the test is performed less than 14 days after the first signs of infection. Age, pre-existing illnesses that cause the immune system to be weakened, or the use of medications such as immunosuppressants (which, for example, are often used to treat arthritis) may reduce the speed at which your body produces antibodies following infection. As a result, the level of antibodies to the SARS-CoV-2 virus in your blood may be below the detection limit of the test. This can lead to a false negative result (i.e. where the test does not identify antibodies even though you have been infected).
We also do not currently know if these antibodies will protect you from getting COVID-19. Therefore, whatever your test result, you should continue to follow government legislation and guidelines. Please do not make any medical or personal safety decisions based on the result of this test.
You will receive information about whether you have antibodies to the coronavirus (and therefore whether it is likely that you have been previously infected), which may be of interest to you.
Your involvement will enable important research to take place to understand the extent of coronavirus infection and enable researchers to determine the risk factors for COVID-19 and its long-term health effects. This, in turn, may help scientists to find new ways to manage the outbreak and to prevent or treat COVID-19.
Participation in the study involves doing a home antibody self-test by obtaining a drop of blood from a finger-prick using a device called a lancet. This produces a small (less than 1mm) prick in the skin.
You may experience slight discomfort when using the lancet (people have described the feeling as a slight sting, much less than you would experience in a typical blood collection with a needle and syringe) and, as with any cut, there is a small risk of infection and/or bruising. However, the equipment is sterile and following the instructions provided in the kit will minimise risk of infection (such as cleaning the area before and after).
The process and products in the kit are used routinely in a wide range of healthcare settings, including for home measurement of blood glucose levels in the management of diabetes.
Yes, this study has received ethics approval from the North West – Haydock Research Ethics Committee, 16/NW/0274.
The information obtained by completing this test will be treated in exactly the same way as the other data we have collected about you. The data will be kept by UK Biobank for many years. They will be provided in a de-identified form (such that no participant can be identified from their data or samples) to approved researchers for medical and other health-related research (including scientists working in other countries and in commercial companies).
We will put the results from all of these studies back into our database for other researchers to use. Scientists must make public the results of all research based on the resource so that everyone can benefit from it. You can find details of research that is being done using the UK Biobank resource and related publications on our website.
We have strict measures in place to protect your confidentiality, which should prevent identifiable information from being used – inadvertently or deliberately – for any purpose other than to support this study:
- We do not include any details that will identify you in any information or samples we provide to researchers.
- We keep any information that might identify you (such as your name and address) separately from other information about you in our database.
- We use advanced computer security technologies to prevent unauthorised access to the computers that hold personal information.
- Our procedures comply with international standards (ISO/IEC 27001) for information security and management. We are externally audited and we comply with the guidance contained in the UK Government’s ten cyber security steps.
- We restrict access to personal information as much as possible, and all research staff working for us sign confidentiality agreements as part of their employment contracts.
The basis on which we process your personal data, which will include any personal data collected for the purpose of the UK Biobank coronavirus self-test antibody study, is explained on our website at https://www.ukbiobank.ac.uk/explore-your-participation/basis-of-your-participation. This includes an explanation of the way in which we protect your data and remove any personal identifiers before making any data available to researchers.
If you would like further information or have any concerns about anything to do with UK Biobank, please refer to our frequently asked questions, or email us at ukbiobank@ukbiobank.ac.uk or telephone our Participant Resource Centre free of charge on 0800 0 276 276 (Monday to Friday 9am to 5pm).
Alternatively, if you would like to write to the person in charge, please send your letter to:
Professor Sir Rory Collins
Principal Investigator, UK Biobank
1-2 Spectrum Way
Adswood
Stockport
Cheshire
SK3 0SA
We shall reply to your letter in writing, unless you enclose your telephone number so that we can call you to discuss your concerns.
UK Biobank Limited (company no. 4978912) is a registered charity in England & Wales (1101332) and in Scotland (SC039230).
IRAS project ID 200778
Version 2.1 02/03/2021
Last updated